Advanced Structural Biology: Crystallography, BioNMR, and Predictive Modeling
Content
Protein structures form the foundation for understanding protein function at a molecular level. X-ray protein crystallography is the oldest and most established method to obtain such structural insights. In addition to elucidating enzyme mechanisms, determining structures of protein complexes is increasingly significant. Crystal structures frequently serve as a basis for resolving larger molecular complexes using CryoEM. BioNMR spectroscopy complements these methods by providing dynamic structural information, particularly valuable for studying proteins in solution and analyzing their conformational changes and interactions.
In this course, we will focus on structural biology in general and explore how protein crystallography and BioNMR can be utilized to obtain protein structures. Additionally, we will discuss protein structure prediction using AlphaFold, including its strengths and limitations. By the end of the course, you should feel confident interpreting structures reported in literature, visualizing and manipulating them using molecular visualization software, evaluating the quality of protein crystal and BioNMR structures, and integrating AlphaFold predictions effectively into your future research.
Core Techniques
In this course, we will work primarily with structural analytical methods both computationally (65%) ~and, to a smaller extent, experimentally (35%).
We will specifically learn and apply these core techniques:
- Visualization and manipulation of protein structures using ChimeraX
- Evaluation and quality assessment of protein structures
- Computational protein structure prediction with AlphaFold (and similiar neuronal networks)
- Protein crystallization using sitting drop methods
- X-Ray diffractions experiments using our in-house X-Ray source
- Solving protein crystal structures by molecular replacement
- BioNMR spectroscopy for studying protein dynamics and interaction
General Organizational Structure
The schedule of the course varies between semesters but generally follows this outline:
- Week 1: Molecular visualization and protein prediction
- Weeks 2-3: Protein crystallization and structure solution
- Week 4: BioNMR: principles, sample preparation, data acquisition, and analysis of protein dynamics and interactions
- Week 5: Study week – applying what you've learned to new problems
- End of Week 6: Journal club focused on your favorite protein structure
Molecular visualization and protein prediction
Molecular structures are inherently complex, and analyzing or presenting them effectively often requires a reduction in complexity. During the first week, we will introduce ChimeraX, a powerful molecular visualization tool, to simplify and effectively illustrate solved protein structures. You will learn how to create clear, informative figures highlighting specific structural features and interactions. Additionally, we will explore ChimeraX to gain deeper insights into protein structure and interactions.
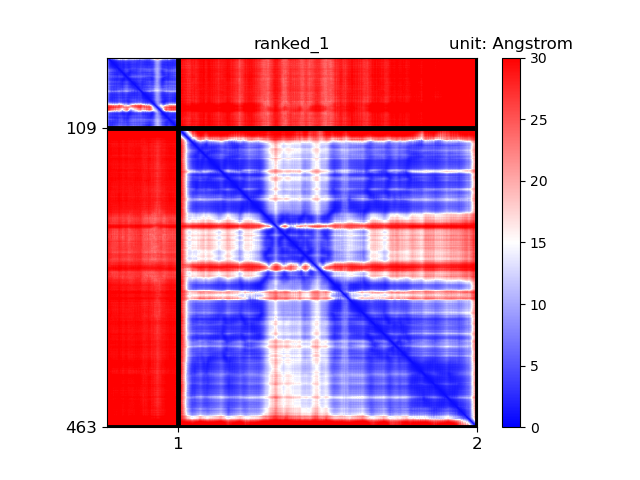
Protein structure prediction has become increasingly accessible and straightforward, largely due to advancements in computational methods such as AlphaFold. However, there are significant pitfalls in interpreting predicted structures. We will discuss the appropriate use of AlphaFold and similar prediction software, emphasizing scenarios where their results should be cautiously interpreted or not fully trusted.
Protein crystallization and structure solution
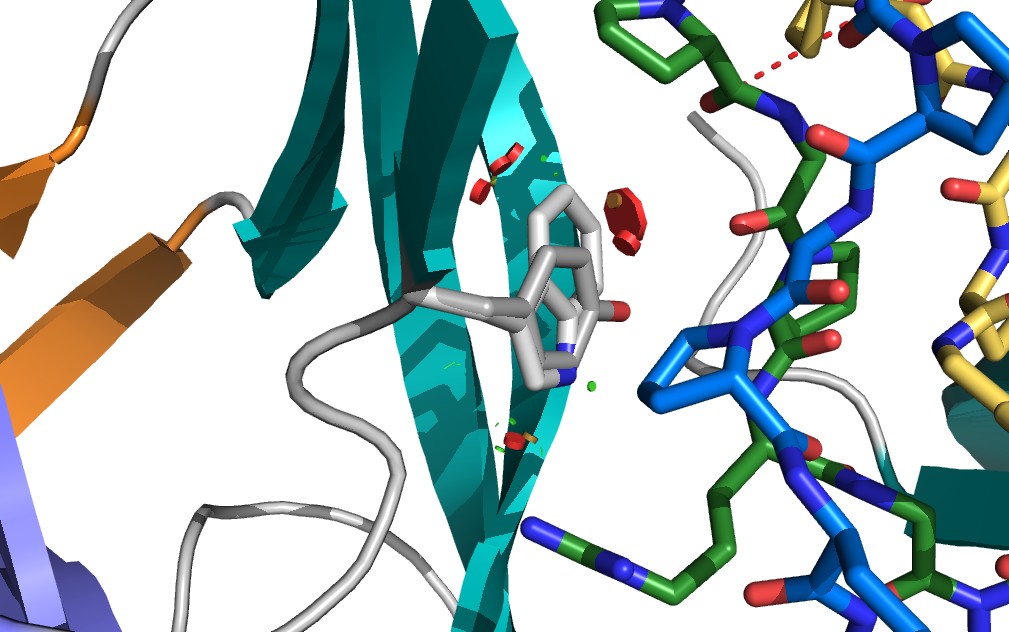
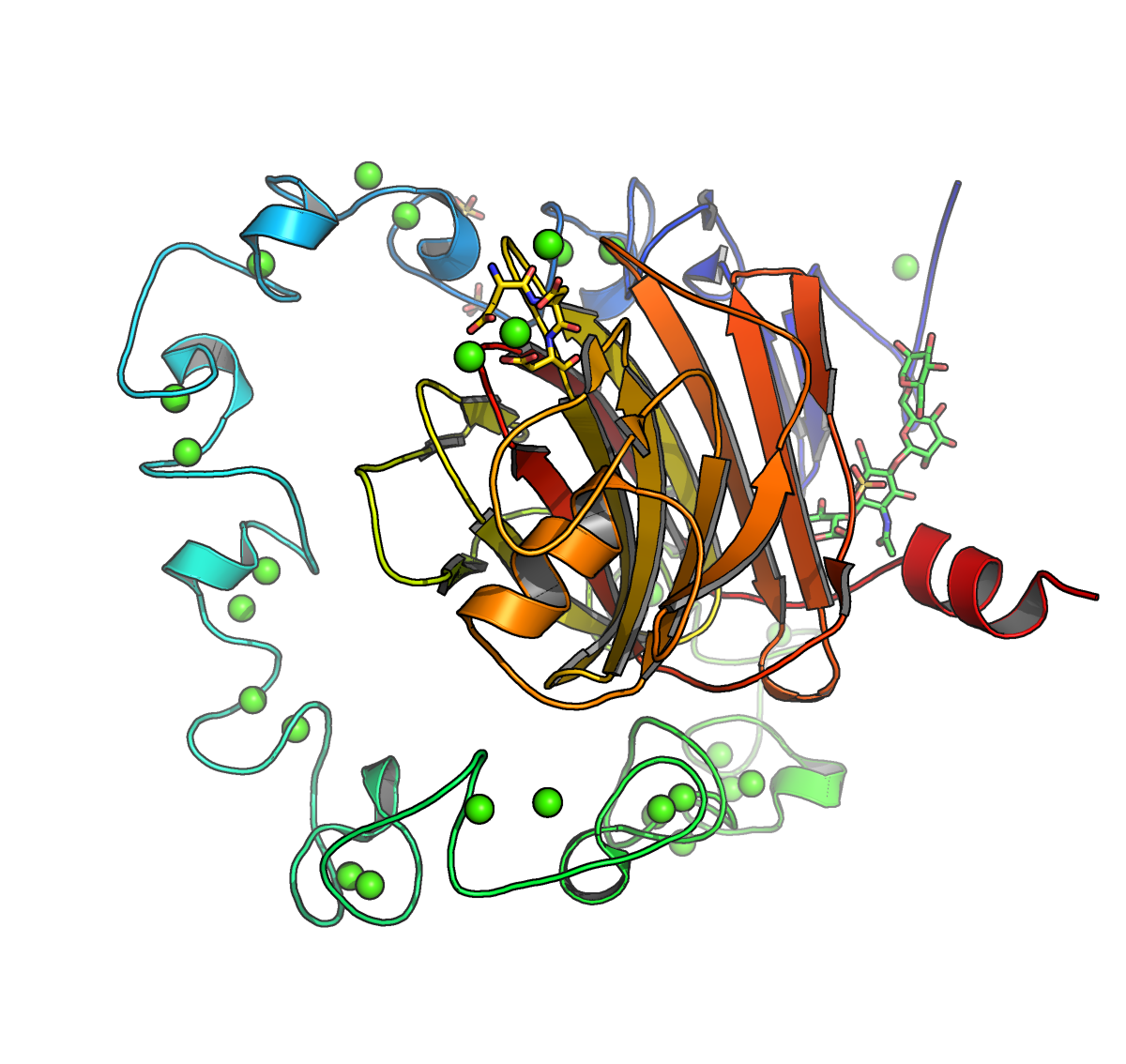
Protein crystallization is a well-established technique for obtaining high-resolution protein structures. In this segment of the course, we will address the practical challenges involved in crystallizing proteins, as well as handling fragile protein crystals. Using our in-house X-ray generator, we aim to acquire high-resolution diffraction images. We will utilize molecular replacement methods to solve the phase problem, followed by iterative rounds of refinement. Ultimately, our goal is to solve the structure of a protein complex of interest, providing valuable insights into its molecular function and interactions.
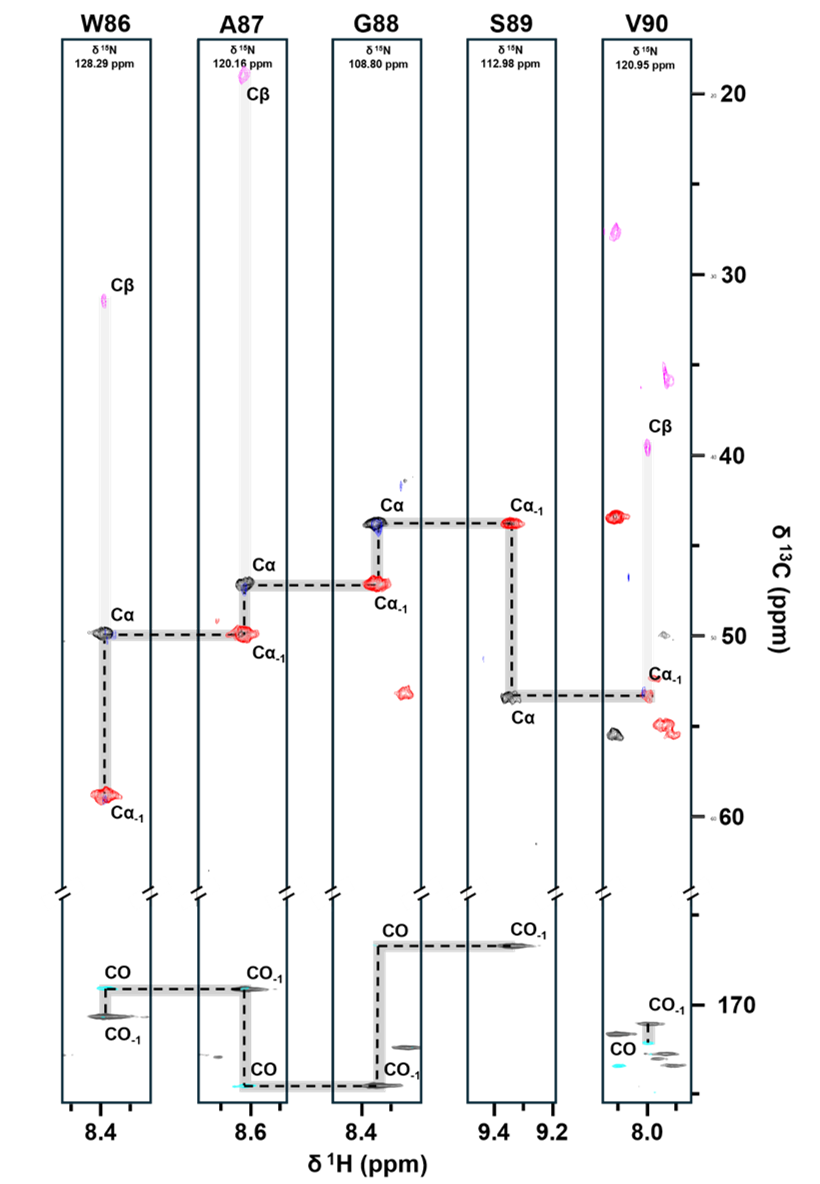
BioNMR: principles, sample preparation, data acquisition, and analysis of protein dynamics and interactions
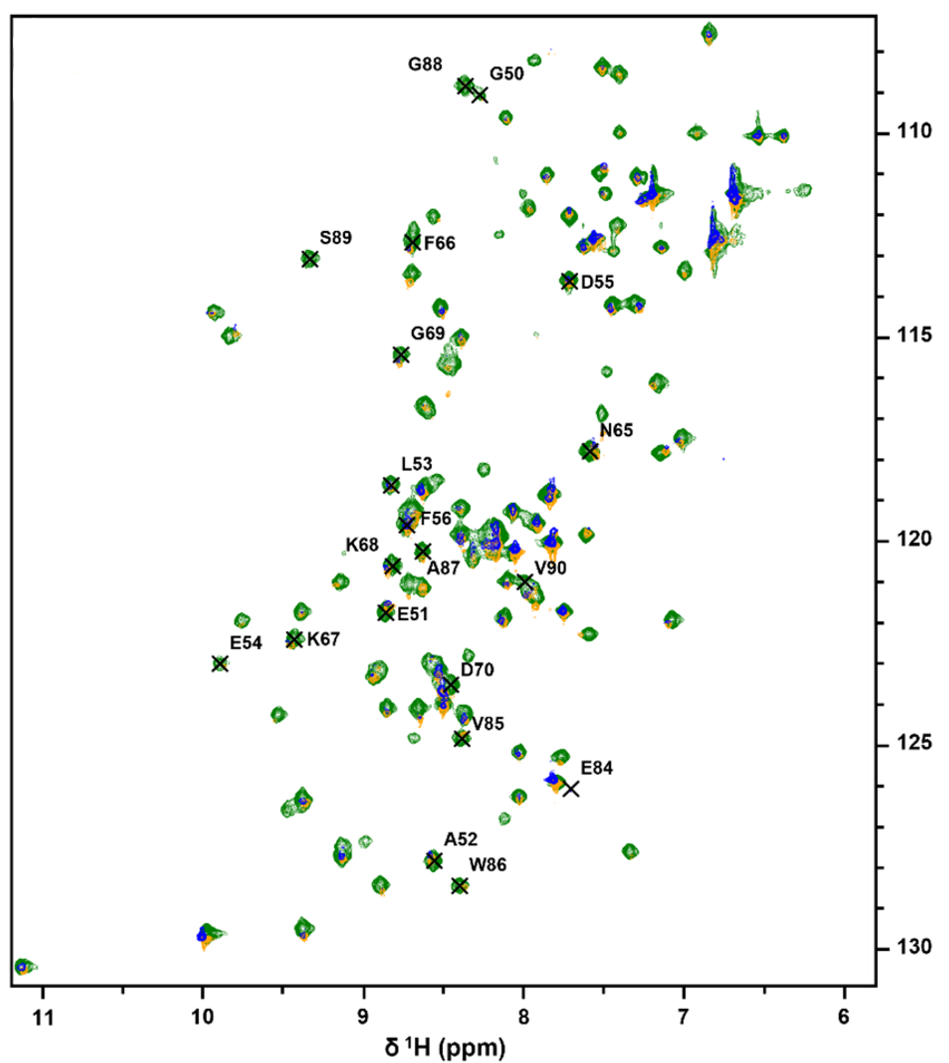
Nuclear magnetic resonance (NMR) spectroscopy is particularly effective for investigating protein dynamics in smaller proteins and identifying interfaces within protein complexes. During this week, we will analyze and annotate NMR data for a small protein, which is also part of our crystallization complex. You will learn the theoretical foundations of BioNMR techniques, including principles of data acquisition and the step-by-step process of spectral analysis and interpretation.