Translation initiation
Translation initiation is the rate-limiting step in protein synthesis and therefore most tightly controlled. The most prevalent mechanism in eukarya is the cap-dependent pathway where a crucial step is the loading of the 43S pre-initiation complex onto the 5’ end of the message and the following scanning process. This is accomplished by eIF4F that consists of eIF4E, the scaffold eIF4G and eIF4A, an ATP-dependent RNA helicase. mRNAs with long and highly structured 5 ´-untranslated regions, e.g. present in ODC or VEGF, are over-proportionally translated upon up-regulation of eIF4F, which is a potential target for the development of anti- cancer chemotherapeutics.
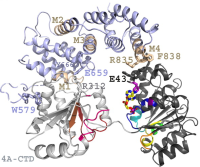
Earlier we reported the structure of the complex formed by yeast eIF4G's middle domain and full-length eIF4A at 2.6-A resolution [1]. This structure allowed insights in the mechanism of eIF4A's activation by eIF4G. In the future our group is interested in the design of new - respectively the improvement of existing- compounds to inhibit this activity.
- Schütz, P., Bumann, M., Oberholzer, A. E., Bieniossek, C., Trachsel, H., Altmann, M., and Baumann, U. (2008) Crystal structure of the yeast eIF4A-eIF4G complex: an RNA-helicase controlled by protein-protein interactions. Proc. Natl. Acad. Sci. U.S.A. 105, 9564–9569
- Dominguez, D., Altmann, M., Benz, J., Baumann, U., and Trachsel, H. (1999) Interaction of translation initiation factor eIF4G with eIF4A in the yeast Saccharomyces cerevisiae. J. Biol. Chem. 274, 26720–26726